Which Compound Is Formed When Aluminum Bonds With Fluorine
A positively charged ion is formed when an atom loses electrons. Ionic compounds are defined as the compounds in which there is complete transfer of electrons from one element a metal to another element a non-metal.

Explain How Fluorine And Aluminum Would Exchange Valence Electrons To Form An Ionic Compound Brainly Com
A Li and Ne B Liand H C Li and Be D Li and B Which compound is formed when aluminum bonds with fluorine.

. Thio show the beyond Ionic bond between aluminum and florida. Warning is that you want to end with um your elements need our cats so they need to lose enoughto have a full outer show or they need to gain enough to have a. No fluorine and sulphur will create a covalent compound because ionic compounds are obtained from non-metal and metal whereas covalent compounds are obtained from only non-metals.
The rest of the fluorite is converted into corrosive hydrogen fluoride en route to various organic fluorides or into cryolite which plays a key role in aluminium refining. The carbonfluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. As the most electronegative element it is extremely reactive.
Aluminium and fluorine combines to form aluminium fluoride. Aluminum fluoride AlF3 CID 2124 - structure chemical names physical and chemical properties classification patents literature biological activities safety. From the given elements two pairs will result in the formation of ionic compounds.
Hydrogen fluoride aluminum fluoride silicon fluorides fluosulfonic acid a solvent catalyst. The following fluorine compounds are widely used. Has more electrons.
With other atoms fluorine forms either polar covalent bonds or ionic bonds. Most frequently covalent bonds involving fluorine atoms are single bonds although at least two examples of a higher order bond exist. The magnesium fluoride has the formula MgF 2.
The magnesium atom gives up 2 electrons to form a magnesium ion Mg 2. C is usually a nonmetallic element. The ionic bond will get form What compound would form between germanium and fluorine if the molecule contains only one.
Ionic compound is formed by the elements. Fluoride may act as a bridging ligand between two. It consists of an aluminum atom surrounded by three fluoride atoms connected with covalent bonds.
The sintering process involves mixing beryl with sodium fluorosilicate and soda at 770 C 1420 F to form sodium fluoroberyllate aluminium oxide and silicon dioxide. Two fluorine atoms bind by single covalent bond only 1 pair of electron is shared between the two. Elements bonds to attain the duplet 2 electron maximum and octet 8 electron maximum electronic configuration which is the stable state.
Is called an anion. It is an inorganic compound having the formula. Almost all other elements including some noble gases form compounds with fluorine.
Simple mix aluminum metal or any compound containing aluminum in which aluminum has weak bonds to the other elements with hydrofluoric acid or another acid along with a fluoride salt like LiF or NaF in water to produce in situ HF. Aluminum fluoride AlF 3 is used to make pure aluminum from oxidized aluminum. Accordingly what type of bond will form between calcium and fluorine.
Al₂O₃ What is the reaction of aluminium and oxygen. 2 lithium and fluorine. Beryllium is most commonly extracted from the mineral beryl which is either sintered using an extraction agent or melted into a soluble mixture.
A transfer of electrons occurs when fluorine and calcium react to form an. What types of bonds will form when fluorine gas reacts with aluminum metal. The chemical formula of magnesium fluoride is MgF₂.
So Aluminum will just give out the 3 electrons to 3 fluorine atoms. Magnesium fluoride is an ionic compound that is made up of one magnesium atom and two fluorine atoms. Covalent bond refers to the bond formed between compounds that result from sharing a couple or more pairs of electrons.
10ー Which pair of elements will most readily form a compound. How do you write the formula for Aluminium oxide. In terms of electron arrangement in the formation of the ionic compound aluminium fluoride the aluminium donates its three outer electrons to three fluorine atoms forming a triple positive aluminium ion and three single negative fluoride ions.
Thus both fluorine and sulphur are non-metals and their compound will be formed only via electrons sharing. Aluminium By mass aluminium makes up about 8 of the Earths crust. So theres a couple of things you have to remember with ionic.
Fluorine Owing to the expense of refining pure fluorine most commercial applications use fluorine compounds with about half of mined fluorite used in steelmaking. Fluorine requires one electron to attain the octet state while aluminum need to give out 3 electrons to attain the octet state. It is the third most abundant element after oxygen and silicon and the most abundant metal in the crust though it is less.
It is one of the strongest single bonds in organic chemistrybehind the B-F single bond Si-F single bond and the H-F single bond and relatively shortdue to its partial ionic character. The two electrons are transferred to fluorine atoms to form two fluoride ions F. 1 aluminium and oxygen.
Fluorine forms a great variety of chemical compounds within which it always adopts an oxidation state of 1. A AIF B AIF C AIF D ALF 12. Okay so the question is asking Thio use orbital notation.
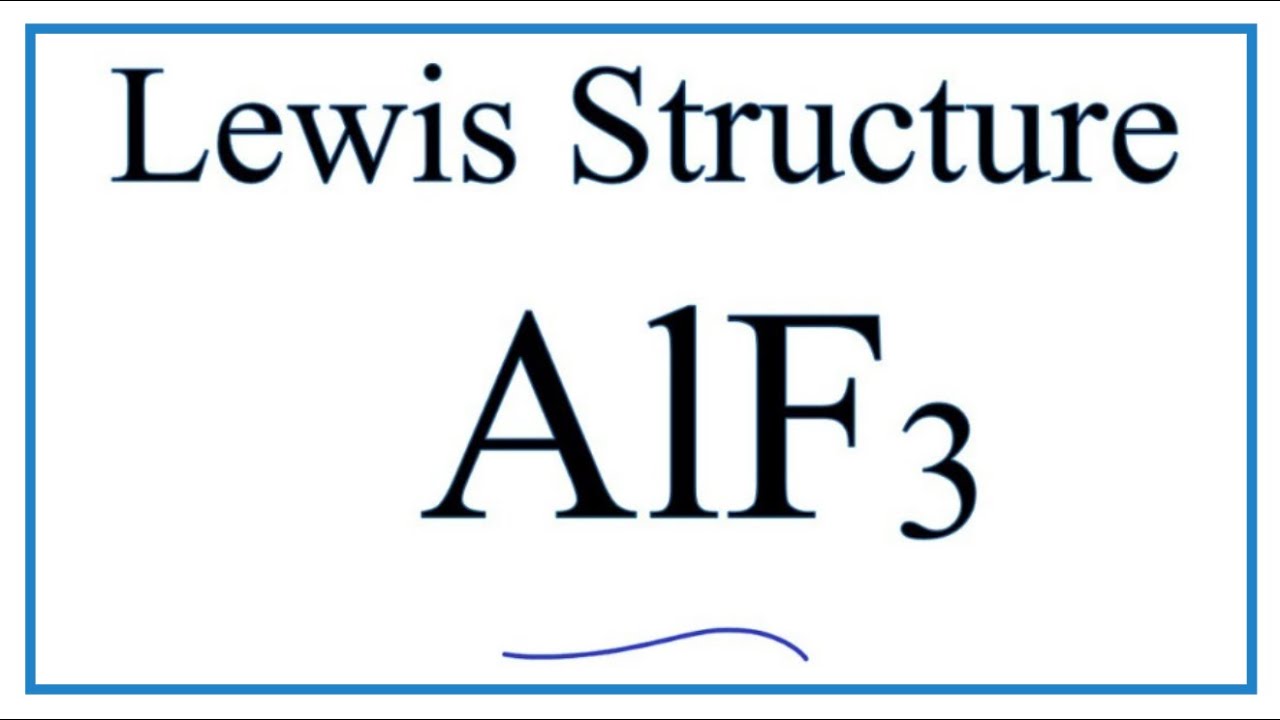
How To Draw The Lewis Dot Structure For Alf3 Aluminum Fluoride Youtube

How To Balance Al F2 Alf3 Aluminum Fluorine Molecular Youtube

Advanced Chemistry Pda Unit 3 Ppt Download
A Question Came In My Exam I E Briefly Explain The Formation Of Ions Of Aluminium And Fluorine I Wrote The Formation Of Al 3 And F 1 Ions After Exam My Classmates Said That
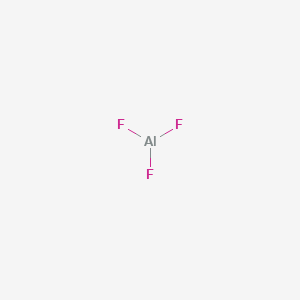
Aluminum Fluoride Alf3 Pubchem

How To Balance Al F2 Alf3 Aluminum Fluorine Molecular Youtube
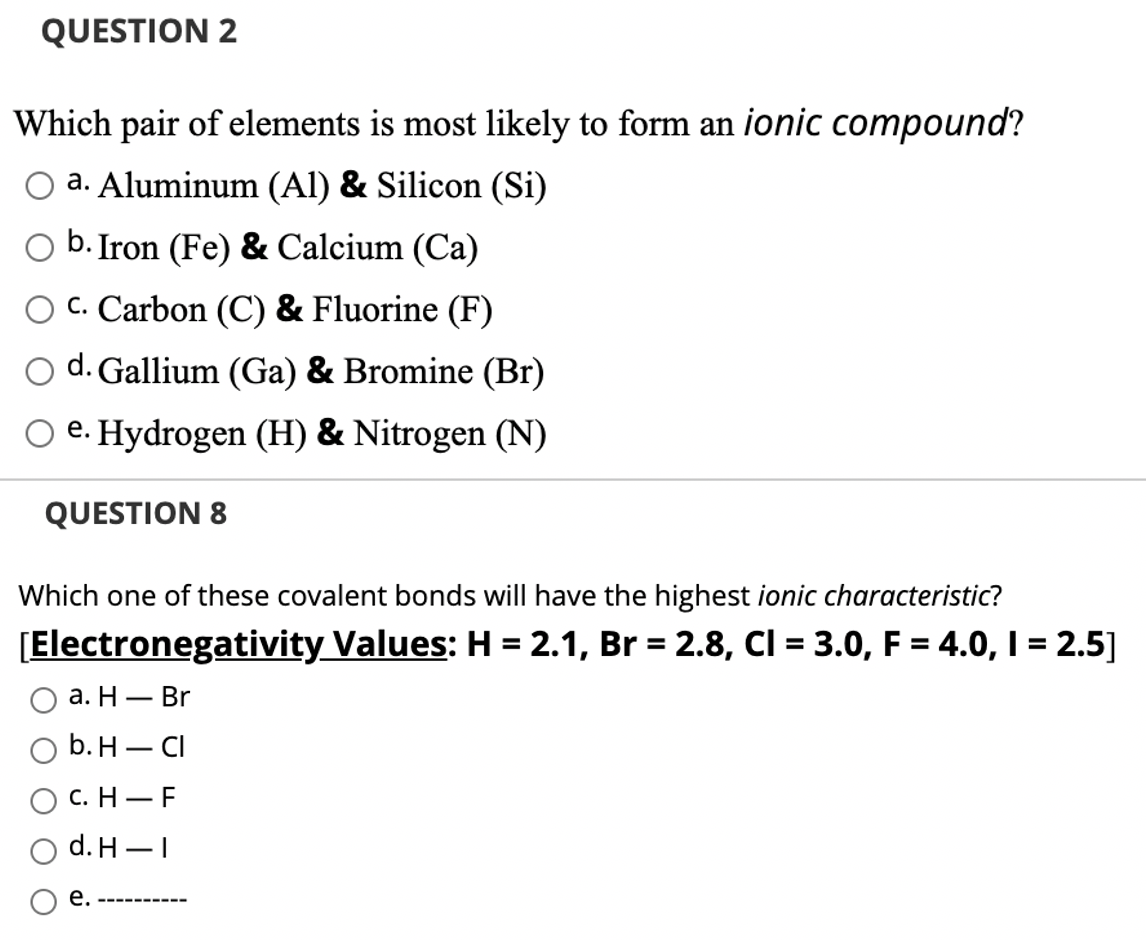
Solved Question 2 Which Pair Of Elements Is Most Likely To Chegg Com

Ionic Compounds An Ionic Compound Results When A Metal Is Attracted To A Nonmetal The Metal Becomes Positive Because They Have A Tendency To Lose Electrons Ppt Download
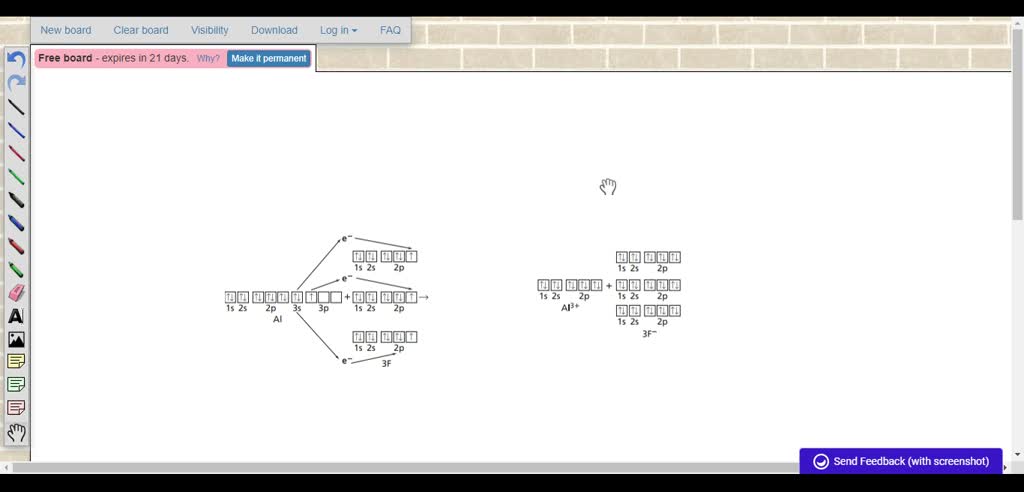
Solved Using Orbital Notation Diagram The Formation Of An Ionic Bond Between Aluminum And Fluorine

Chemistry Chemical Bonding 18 Of 35 Lewis Structures For Ionic Comp Aluminum Oxide Al2o3 Youtube

Aluminum Fluoride Formula Structure Properties Manufacturing Study Com

Bell Ringer Caf2 Al2s3 What Should We Call These Compounds Ppt Download

Aluminum Fluoride Formula Structure Properties Manufacturing Study Com

How To Write The Formula For Aluminum Fluoride Alf3 Youtube
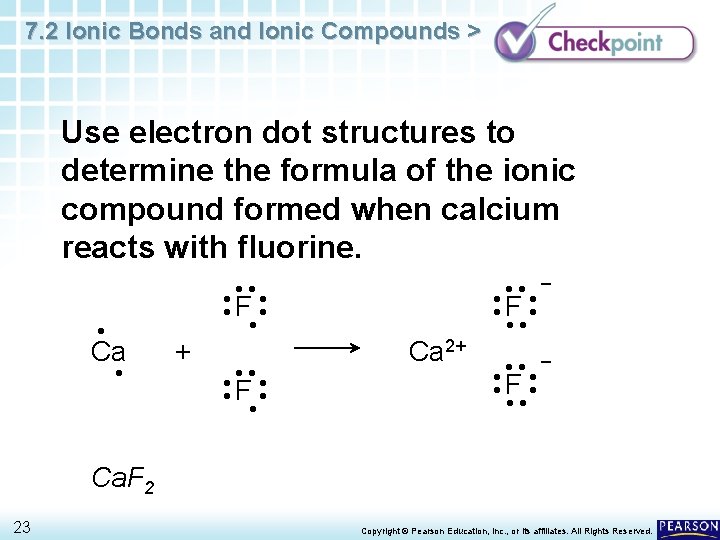
7 2 Ionic Bonds And Ionic Compounds Chapter

Lewis Structure Of Alf3 Aluminum Fluoride Youtube
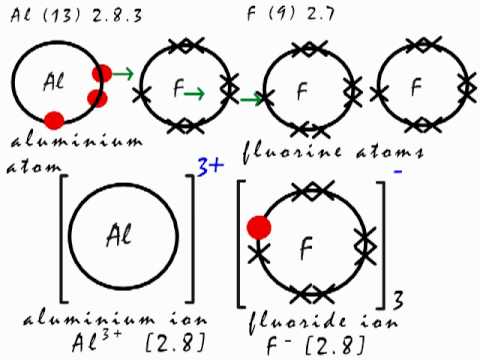
This Is How The Ionic Bond Forms In Aluminium Fluoride Alf3 Youtube

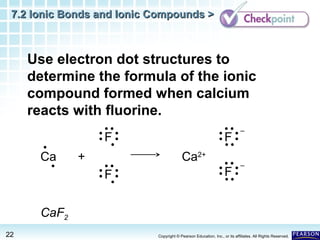
Comments
Post a Comment